Nothing exists except atoms and empty space; everything else is opinion.
-Democritus (460 BC – 370 BC)
The above quote by Democritus may not be exact – seeing from the eye of Modern Physics. But it does make sense. Everything we see around us is made of Atoms. The computer screen you are looking right now is made of atoms. The eye you are using to look at the computer screen is made of atoms. The brain you are using to process the information given to you by eye is made of atoms.
You are made of atoms. And you are reading about atoms. Basically – here – ATOMS ARE READING ABOUT ATOMS as Neils Bohr says –
A physicist is just an atom’s way of looking at itself.
– Neils Bohr
Is it getting interesting?
Well, Studying about atoms isn’t only interesting – It is very important. If you want to understand the universe – you must understand atoms.
Why?
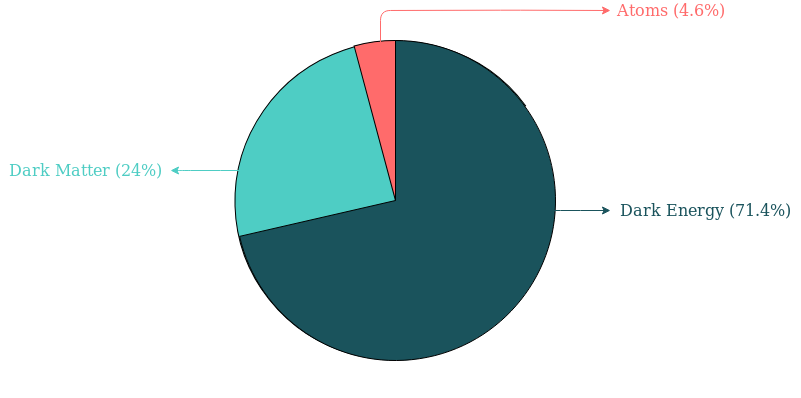
– Because about 4.6% of the universe – and almost all things around us are made of atoms (or parts of atom). You can see the following chart for more information. The data was taken from NASA.
Here,
- Smallest i:e red part corresponds to atoms (or parts of atom).
- The biggest part thats coloured dark blue corresponds to Dark Energy.
- Light blue corresponds to Dark Matter.
So it is logical to spend some time of your life to understand about atoms if you want to understand the universe or the things around you. And that’s what many scientists have been doing.
And that’s what Thapa Poudel Aakash wanted to do – as he asked following question in our Facebook group.
We value your privacy.
Here’s your answer Mr Aakash.
In this article, we will explore how scientists were able to know about the internal structure of atoms. We will be discussing some of the most important models(structure of atoms) that were proposed. We will even know a little about the technique that astrophysicists use to know about the atoms that make stars and distant celestial bodies. And lastly – we will see some images and videos from Scanning Tunneling Microscope.
Are you excited? Let’s start.
Table Of Contents
The concept of Atom is very old. It can even be found in ancient cultures of Greece and India. However, the first scientific evidence of atom was provided by John Dalton in the 1800s. By the 19th century, it was reliably known that matter is made of atoms.
In 1896, J.J. Thomson found that the atom had an inner structure. After this, physicists started to think about the inner structure of the atom. The following story is about physicists theorizing about the inner structure of the atom.
Thomson’s Atomic Model
Sir Joseph John Thomson, in 19th century, did some experiments with Cathode Ray Tubes.
Cathode Ray Tubes are simply glass tubes from which all air have been taken out. If you are curious about how they look. You can see the image below. The electronics on the left-hand side are there to emit a beam of particles. The properties of those particles were unknown at the time.

J.J Thomson wanted to know about the particles that get emitted from it. For this, he put electric plates and magnets on the side of the tubes. You can see the result below (with a magnet).
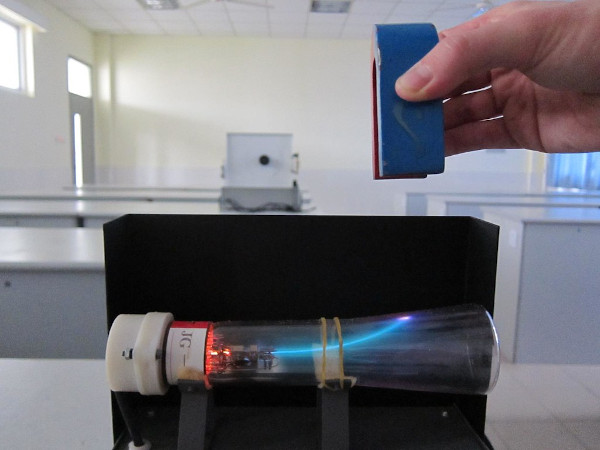
As you can see in the picture – the ray bends in the presence of a magnetic field.
J.J Thomson also placed electric plates on the sides. He found that the ray bends towards a positively charged plate and bends away from a negatively charged plate. This meant the particles repel negative charge and are attracted towards positive charge.
As we all know – like charges repel and unlike charges attract – J.J Thomson concluded that the particles were negatively charged.
Combining the results of the experiment – J.J Thomson found that the particles that are emitted have 2000 times less mass than the mass of Hydrogen atom.
This was and is very interesting and was somewhat unbelievable.
For a long time, humans thought atom to be the smallest and indivisible thing.
But J.J Thomson found something very small than an atom.
Could anything at first sight seem more impractical than a body which is so small that its mass is an insignificant fraction of the mass of an atom of hydrogen?
– J.J. Thomson.
J.J Thomson repeated the experiment keeping different metals as electrodes and got the same result every time. This means atoms of every element contained these small negatively charged particles.
From these facts, J.J Thomson concluded
- Atoms are not indivisible. They contain smaller particles that make it up.
- The particles in cathode rays are negatively charged.
- The mass of particles in cathode ray is about 2000 times less than the mass of hydrogen atom.
- So atoms have very small particles that are negatively charged.
Later, the particles were named “Electrons”.
J.J Thomson knew that atom is electrically neutral i:e atom has no charge. That means if it has negatively charged particles – it must have positively charged particles too. So J.J Thomson told that Atom is made up of electrons and positive charges.
With these – J.J Thomson made his atomic model.
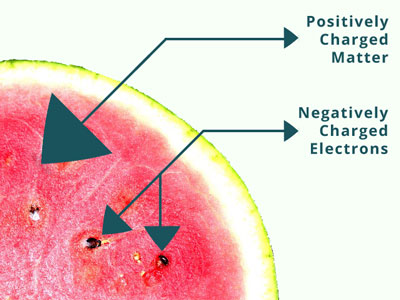
In 1998, J.J. Thomson proposed that atom is spherical in nature. He thought the sphere to be positively charged with negatively charged electrons in it just like seeds are embedded in watermelon.
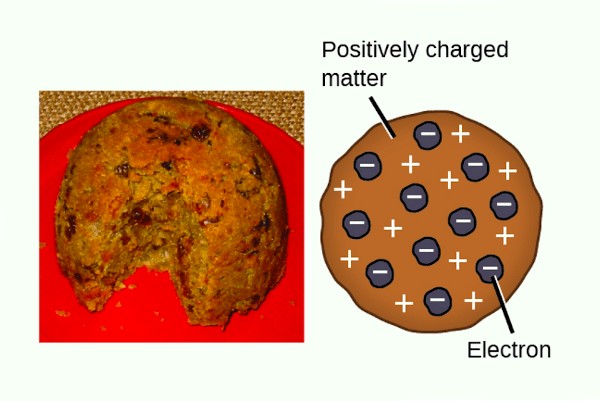
J.J Thomson’s atomic model is also called as Plum Pudding Model. Below illustration explains the reason.
Fun Fact: J.J. Thomson obtained Nobel Prize in Physics in 1906 for the discovery of the electron and his work on the electrical conductivity of gases.
But this model was found to be defective later on.
Especially by the famous Rutherford Alpha-Particle Scattering experiment. The experiment done by Rutherford showed that the positive charge of an atom is in the centre of an atom.
Rutherford Atomic Model
As J.J Thomson did – Rutherford also conducted an experiment to understand more about atoms.
Rutherford’s experiment is widely known as Rutherford’s Alpha-Particle Scattering Experiment. It’s because in his experiment – he shot atoms with Alpha-Particles.
Alpha-Particles are positively charged particles that come as a byproduct during radioactive decay.
In the experiment – It was found that most Alpha Particles could pass through the atom. But some particles couldn’t pass right through as shown in the figure below.
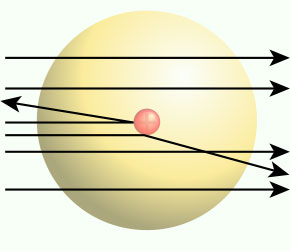
He found that the Alpha-Particles couldn’t pass through the centre of the atom. It could only mean that the atom has something in the centre.
Alpha particles are positively charged. And it was known from the experiment that the centre of atom repelled Alpha particles. Rutherford concluded that the centre of the atom is positively charged as like charges repel.
He also found that Alpha-Particles could pass easily in most of the cases. It indicated that most of the atom is empty space.
Rutherford from his experiment concluded,
- Most of the atom is empty space.
- The size of the atom (diameter) is about 10-10m.
- All positive charge of the atom is in the centre of the atom.
- Negative charge i:e electron revolves around the nucleus (centre) of the atom.
Imagine 10-10m.
Look at your hair. How thin is it?
Very thin, right? It’s about 500,000 carbon atoms side-by-side. Now imagine how small a single carbon atom is!
On the basis of the Alpha-Particle scattering experiment, Rutherford made his own atomic model in 1911 A.D.
Rutherford Atomic Model described the atomic structure as below
- At the centre, there is a small nucleus which is positively charged.
- The nucleus contains most of the mass of an atom.
- Electrons revolve around the nucleus.
Rutherford Atomic Model is used in the logo by many scientific organizations. Some of the organizations that use Rutherford Atomic Model in their logo are
- The United States Atomic Energy Commission
- The International Atomic Energy Agency
- PhysicsDB.com – Yeah, we also use Rutherford’s Atomic Model in our logo. Can you find Rutherford Atomic Model in our cover picture below?

Drawbacks Of Rutherford Atomic Model.
According to the Rutherford Model, atoms revolved around the nucleus.
We all know that if charge revolves, it must lose some energy. And if charge loses energy, it can’t revolve as before.
This means a revolving electron must lose some energy. After losing energy, the electron’s orbit must decrease. And after enough time, the electron should fall into the nucleus. This would make atoms unstable. But this is not true. Atoms are stable in nature.
Rutherford couldn’t explain Atomic Spectra too. Later, Bohr Atomic Model explained it.
But before jumping to Bohr’s Atomic Model, let’s discuss Atomic Spectra first.
It’s important because it had a huge influence on the development of Quantum Mechanics.
Atomic Spectra
We shall look restrict ourself to the emission spectrum of some atoms here (for simplicity). Emission spectrum is basically the collection of lights that an element or molecule can emit when they make the transition from a higher energy state to lower energy state.
Let’s see the spectrum of visible light. It means the collection of light that’s visible to human eyes. Here’s an approximation.

Now, let’s see the emission spectrum of Hydrogen below:

As you can clearly see – Hydrogen only emits certain light. If you compare this emission spectrum to the above spectrum of visible light – you can know most kinds of light are missing easily. Since Hydrogen has only one electron – it is simple in nature. And that’s why every atomic structure class discusses Atomic Spectra of Hydrogen.
Let’s look at the emission line of an element with more electrons – Iron.
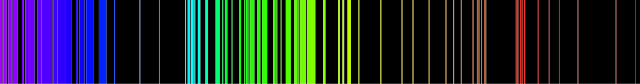
As we can see in the emission spectrum, Iron can emit more kind of light – but still – it can only emit certain kind of light.
You can again compare this emission spectrum to the above spectrum of visible light to know the kind of light that’s missing.
One interesting thing about Atomic Spectra is that – it’s unique for every element.
Just like fingerprints are unique for humans. So fingerprints can be used to identify humans.
Atomic Spectra are unique for elements. So Atomic Spectra can be used to identify elements. This technique of identifying element through Atomic Spectra is called Spectroscopy.
In fact – Astrophysicists use this technique to find out the elements that make up stars or even the atmosphere of Jupiter.
Isn’t it interesting?
If you want to see the emission spectrum of more elements – you can view this page of Penn State Public Research University ‘s Periodic Table. If you click on elements – it will show you it’s emission spectrum. You can see that every emission spectrum is discrete – no element has a continuous emission spectrum.
But why is Emission Spectrum discrete and not continuous?
Rutherford told electron could revolve around atoms in any orbit. It means electrons can have any amount of energy. It means Atoms could radiate any frequency of light.
But that wasn’t the case. In reality – atoms can radiate only fixed frequency of light.
Rutherford couldn’t explain it.
Then, Niels Bohr – a Danish Physicist and a Nobel Laureate presented a new atomic model. This model explained that atoms could revolve around the nucleus without losing energy, explained Atomic Spectra and many other questions about Atomic Structure.
Bohr’s Atomic Model

- The electrons only revolve in orbits where the angular momentum i:e amount of rotation of the electrons is equal to nħ.
Here,
- n is known as the principal quantum number and takes natural numbers as it’s value. Thus n=1,2,3,4..and so on,
- ħ is reduced Planck’s constant whose value is equal to
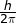 .
.
What this means is, the angular momentum of electrons can only be 1ħ,2ħ,3ħ… and so on. It can’t be 1.1ħ,1.2ħ,2.3ħ, etc. This is called called the quantization of angular momentum.
- The orbits such that the angular momentum of an electron revolving there is equal to nħ are called stationary orbits.
- The electrons can revolve around the nucleus in stationary orbits without losing energy.
- The electrons absorb energy (light) when it goes from a lower orbit to a higher orbit. When light is absorbed by an electron, it gains the energy of the light. When it gains energy – it jumps to higher energy level i:e higher orbit.
- The electrons radiate energy (light) when it goes from a higher orbit to a lower orbit. When an electron gives light, it loses energy. When it loses energy – it jumps to lower energy level i:e lower orbit.
We need to know that, electrons don’t absorb or radiate every kind of light. (Remember something?)
This is because electron absorbs or radiate energy just enough to change their energy levels. This is because as electrons gain or radiate energy – they change their orbit too. If they exchange any kind of light then they should be able to be anywhere. But that’s not possible. They can only be in the stationary orbits. That’s why they don’t absorb or radiate every kind of energy.
Let’s convert these words into math.
Let’s suppose the higher energy level has energy “Ehigh“. Also, let’s suppose the lower energy level has energy “Elow“.
If we suppose that electron absorbs energy – then electron must jump from lower orbit to higher orbit. Since it was in the lower orbit – The electron had energy “Elow“. After jumping to higher orbit – Its energy became “Ehigh“.
Thus the energy gained by the electron must be equal to Ehigh-Elow. – Quick Maths.
The energy of light is equal to hf.
Where,
- h is Planck’s constant, and
- f is the frequency of light.
As the light got absorbed by electron – the energy that the light had – gets gained by the electron so,
Energy gained by electron=hf
So,
Ehigh-Elow=hf
Thus,
![]()
So the electron only absorbs or radiate light which has a frequency equal to ![]() .
.
The fact that – Electrons emit or absorb only certain frequency of light is explained by Bohr’s Atomic Model.
Let’s see emission spectra of hydrogen again.

As we can clearly see – electrons don’t emit every kind of light.
Back to Bohr’s Atomic Model,
The orbits are at a distance such that the angular momentum of the electron is equal to nħ.
The angular momentum of the body with mass “m” revolving in a circular path with radius “r” with velocity “v” is equal to mvr. So,
![]()
This is also called as Bohr-quantum condition.
The value of ħ is h/2π. Putting the value,
![]()
and simplifying the equation by dividing both sides by mv,
![]()
Using this equation, Bohr was able to find all possible discrete radius of orbits.
Bohr successfully calculated the radius of the first orbit of Hydrogen atom too. The value is nowadays denoted by a0 or sometimes rBohr. We won’t go into the derivation of this equation in this article because it will make this article very long. But we shall know that he was also able to calculate it as –
![]()
Here,
- a0 is the Bohr Radius i:e the smallest possible radius for the path of an electron around the nucleus.
- ϵ0 is the permittivity of free space,
- ħ is the reduced Planck’s constant,
- me is the electron rest mass,
- e is the elementary charge,
- α is the fine structure constant.
He found the value as 0.0529 10-9 m.
This might seem hard to understand. But below animation will probably make it easy.
As you can see in the animation – the blue rotating particle is an electron, the green wave is energy(light) and red at the centre is the nucleus.
- The electron is revolving in a fixed stationary orbit.
- When it loses energy due to external agency – electron jumps to lower orbit.
- When it gains energy due to external agency – electron jumps to a higher orbit.
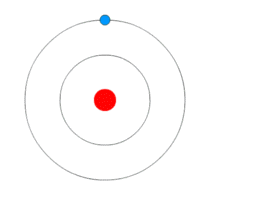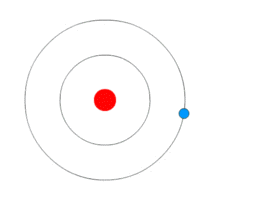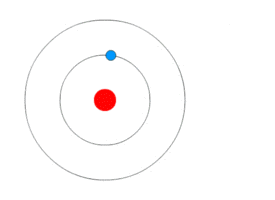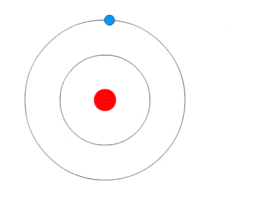
Bohr Atomic ModelBy Kurzon – Own work, CC BY-SA 3.0, Link
This model is also called as Planetary model because of similarity in the movement of planets around the sun. Look the animation above, it does look like planets around the sun.
Drawbacks Of Bohr’s Atomic Model.
Though Bohr’s atomic model answered many questions, it still had many defects.
- For example, Bohr’s Model violated Heisenberg’s Uncertainty Principle.
Heisenberg’s Uncertainty Principle says that – It is impossible to know both position and momentum of an electron at the same time, but Bohr’s Model tells it’s possible to know both position and momentum. - We discussed the Atomic Spectra and its explanation by Bohr’s Atomic Model. Bohr did give an idea about Atomic Spectra but it could only predict spectra of small atoms like Hydrogen. It couldn’t predict the spectra of bigger atoms or atoms.
- Bohr’s Atomic Model couldn’t explain how spectral line splits into multiple spectral lines when kept in Magnetic Field (Zeeman Effect), Electric Field (Stark effect). , etc.
Many improvements to Bohr’s Model has been made to explain the things that Bohr couldn’t explain.
Sommerfeld Atomic Model is one of them. Sommerfeld told that electrons don’t revolve in circular orbits, but orbit in an elliptical path. Circular orbits are seen in special case only. He also told that mass of electron changes relativistically with velocity as it moves. So it is also called the Relativistic Atom Model.
Then Vector Atom Model was created to correct it even more. Vector model introduced even more Quantum numbers and blah blah…
Another famous model is the Quantum Mechanical Model. It is also known as the Electron Cloud Model because that model explains – You can’t know both position and momentum of an electron at the same time. So instead of orbits like in model of Rutherford, Bohr, etc – The Electron Could Model says – there exists cloud around the nucleus where the probability of finding the electron is distributed. We can see that below.
Here the black region is the electron cloud. As you can see, there is a dark region close to the nucleus and light region away from the nucleus. The chances of finding an electron in the dark region are high and the chance of finding the electron in the light region is low. So the electron is likely to be in the darker place but that’s not sure. It could be anywhere.
Getting in details about this model will require a lot of mathematics. Khan Academy has few articles about it.
Many improvements were done and yes – they are interesting. But it isn’t possible to explain all of them at once – here. So I think we need to stop here
But as promised before, here’s an image of Atoms of gold taken by Scanning Tunneling Microscope. Scanning Tunneling Microscope works using the concept of Quantum Tunneling.
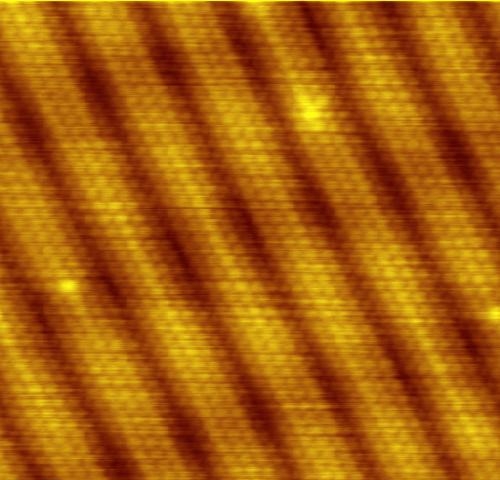
Want some bonus? Here’s a video that was taken using a Scanning Tunneling Microscope. Thanks to IBM.
This is the world’s smallest movie and was made by moving Carbon monoxide molecules which is two-atoms molecules.
If you are curious about how they made it, you can see the following video or read this Wiki.
Frequently Asked Questions (FAQ)
- Who discovered the structure of atom?
– We can probably give the credit to J.J Thomson. Because J.J Thomson was the first person to scientifically suggest that atoms had structure too. Before J.J Thomson, people used to think the atom had no structure at all. - What is the basic structure of an Atom?
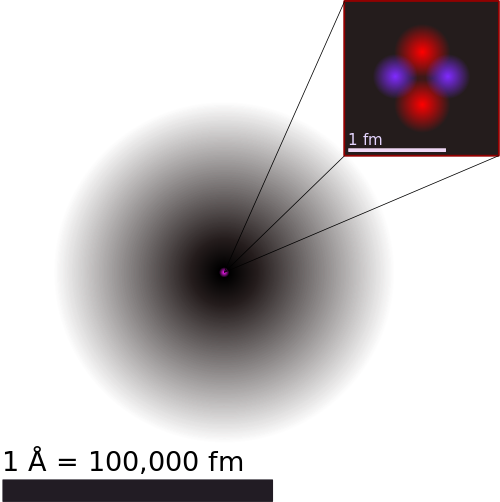
– Atom has a nucleus in the centre and electrons revolve around the nucleus. The nucleus is positively charged and the electrons are negatively charged. - What is the real structure of Atom? or What are the internal structure of Atom?
– Atom has a nucleus in the centre and electrons revolve around the nucleus.
The nucleus is further made up of Protons and Neutrons. The Protons are positively charged while Neutrons are neutral i:e they have no charge. This makes the nucleus – as a whole – positively charged.
The electrons are negatively charged. - How is the structure of Atom with diagram?

By User:Yzmo – Own work, CC BY-SA 3.0, Link Here – the red ones are protons and blue are neutrons. They are located in the centre. Around them is electron cloud where electrons revolve.